Research and Development
Pipeline and project portfolio
The Respiratorius project portfolio includes projects targeting cancer
Pipeline and project portfolio
The Respiratorius project portfolio includes projects targeting cancer
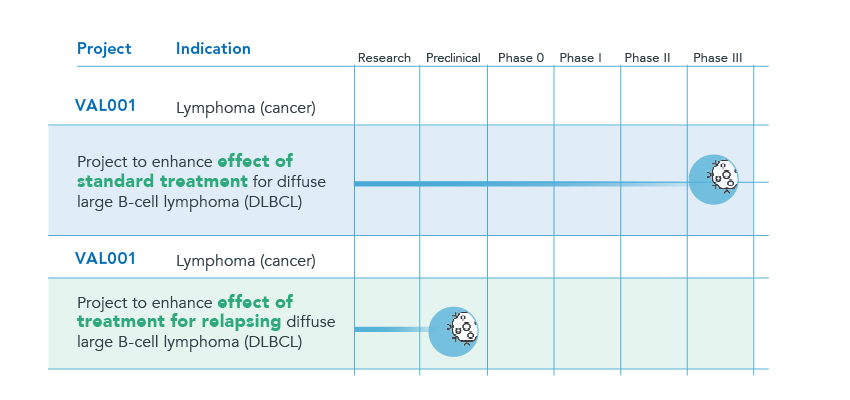
Below is a brief overview of Respiratorius’ primary projects
VAL001 is a drug candidate that has shown clearly promising experimental and clinical data against diseases such as diffuse large B-cell lymphoma (DLBCL), the most common type of non-Hodgkin’s lymphoma. The Company has successfully completed a phase I/IIa study that was conducted at Skåne University Hospital in Lund, Uppsala University Hospital, and Norrland University Hospital in Umeå.
Results from the phase I/IIa study show significantly increased survival (1-year and 2-year survival) among patients treated with VAL001 prior to treatment with R-CHOP, compared with patients treated with R-CHOP alone. Comparative data were taken from the Swedish Lymphoma Registry with a matched reference population of patients who were treated between 2010 and 2015. The results from the phase I/IIa study also demonstrate specific effects through increased levels of CD20, which may likely be beneficial in patients treated with Rituximab.
As part of the scientific advisory process with the EMA’s Scientific Advice Working Party (SAWP) regarding clinical strategy, VAL001 was assessed as meeting the criteria to directly begin a Phase III study. Inclusion of about 700 patients should be satisfactory for such a study to be able to serve as a basis when applying for marketing authorization. VAL001 for the treatment of DLBCL received orphan drug status in Europe and the US, and patents were granted in the EU, the US, Canada, Japan and Korea. There is also a patent application for the formulation of VAL001 as well as for relapsing DLBCL.
Respiratorius AB (publ) develops candidate drugs with the goal of launching drugs to treat aggressive lymphoma (cancer).
Respiratorius AB
Medicon Village
Scheeletorget 1, 223 81 Lund, Sweden
info@respiratorius.com
+46 70 922 41 40

To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions.
